Introduction
This section describes various types of electrical connectors and provides a list of recommended manufacturers.
You will learn about:
- Electronic Connectors and their purpose
- Common terminology used with electronic connectors
- Different types of electronic connectors
- Methods for terminating electronic connectors
- Additional important information
Chapter 1: Understanding Electronic Connectors
Electronic connectors are essential components that join electronic circuits together. They play a vital role in assembling, installing, and powering electrical devices across various applications, including industrial equipment, consumer electronics, communication systems, and household appliances. Though often overlooked, connectors ensure electronic products function properly. While some connectors create permanent connections, most are designed for temporary and easy detachment.
An electrical connector consists of two main components: contacts and housing (commonly called plugs or receptacles). The housing holds the terminals in place, maintains stable connections, and provides insulation to prevent interference with other electronic components, thereby avoiding short circuits. Plugs and receptacles protect terminals from environmental factors and are typically made from insulating materials like molded plastics or ceramics.
Depending on the application, connectors may include additional features. Keyed connectors ensure proper orientation during connection to prevent mismating. Some feature locking mechanisms to prevent accidental disconnection, while others are sealed for underwater use.
Connector terminals are the contact pins that create continuous electrical pathways between circuits. These are made from conductive materials such as brass, phosphor bronze, beryllium copper, and high-copper alloys.
Chapter 2: Terminologies Used in Electronic Connectors
Understanding electronic connector terminology is crucial for selecting the right components for your systems. These terms describe key properties, construction features, and performance metrics that determine connector types and compatibility. Familiarity with these terms aids in design decisions, procurement, and troubleshooting across electrical engineering, electronics assembly, and industrial automation applications.
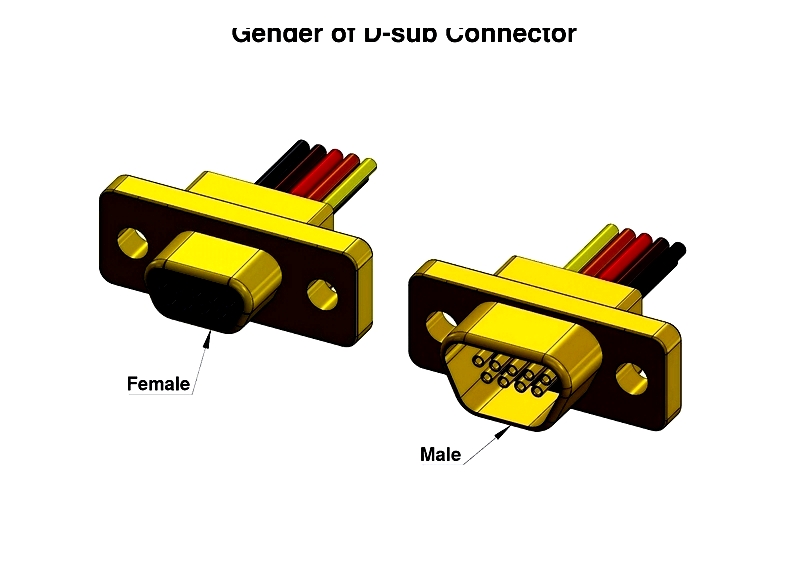
Gender
In electronic connectors, gender classifies mating connectors based on size, shape, and pin configuration. The male connector (plug) contains pins that insert into a female connector (receptacle or socket). Female connectors have socket holes with terminals attached to wires or cables. Proper gender selection ensures secure mating and electrical integrity in various electronic devices and PCB assemblies.
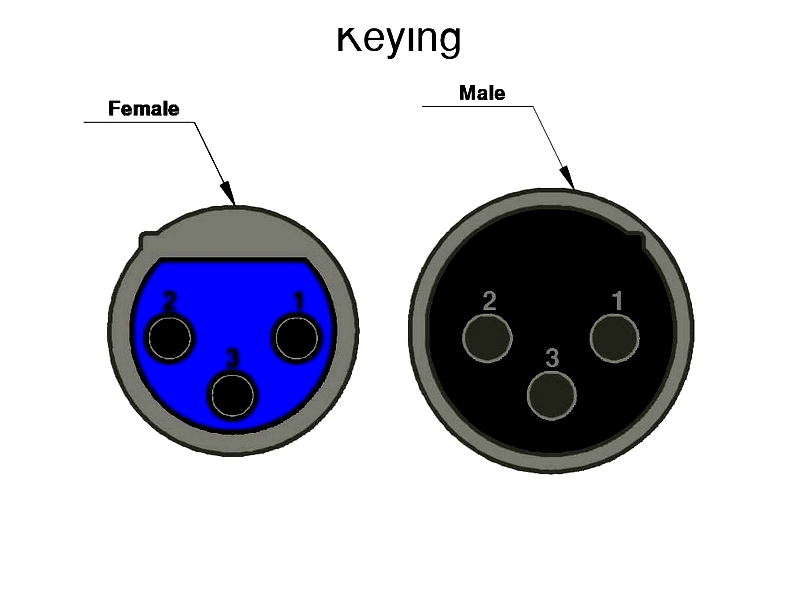
Keying
Keying prevents incorrect mating that could cause electrical failures or mechanical damage. This design feature uses physical elements like keys or notches to ensure connectors only mate in the correct orientation. Keying is particularly important for multi-way or symmetrical connectors where misalignment could damage components.
Locking Mechanisms
Locking mechanisms secure mated connectors to prevent accidental disconnection. Common types include push-pull, bayonet couplings, and screw couplings, chosen based on application needs. These mechanisms provide vibration resistance and environmental sealing for demanding industrial, automotive, or aerospace environments.
Number of Contacts
The number of contacts refers to the total pins or terminals in a connector, ranging from simple 2-pin designs to complex connectors with over 200 contacts. This count depends on device requirements and signal complexity, affecting connector size and signal routing capabilities.
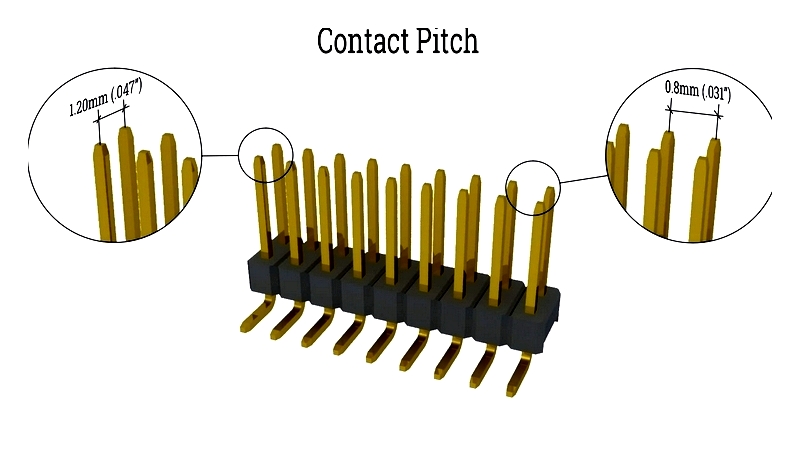
Contact Pitch
Contact pitch measures the center-to-center spacing between adjacent pins, typically in millimeters or inches. This spacing affects connector compatibility with PCBs and cables, influencing signal integrity and miniaturization. Larger pitches reduce arcing risk, while smaller pitches enable high-density layouts.
Proper contact pitch calculation ensures optimal electrical performance and design compatibility.
Pin Numbering
Pin numbering standardizes terminal identification within connectors. Numbering schemes (left-to-right, top-to-bottom, or industry-specific) prevent wiring errors in complex connectors and cable assemblies, essential for automotive wiring and PCB layouts.
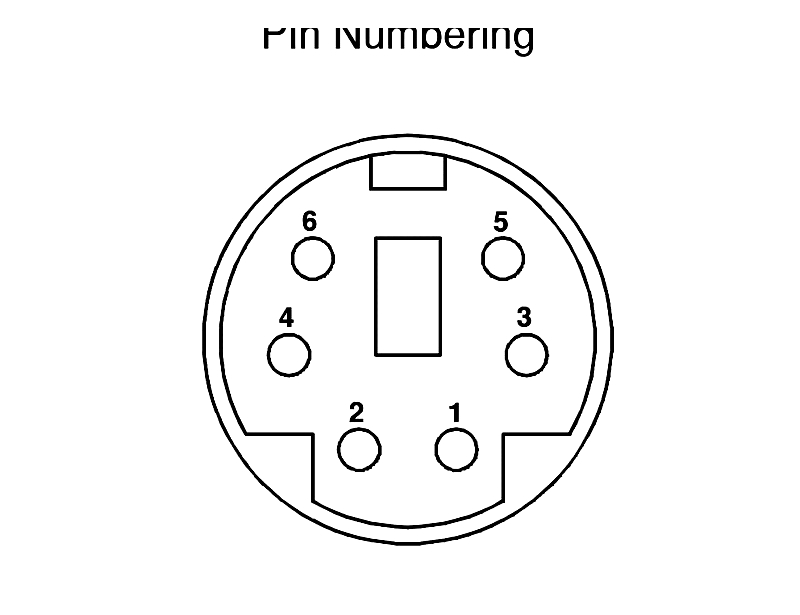
Pin Sequence
The pin sequence determines the order in which contacts engage during mating. Proper sequencing ensures ground or shield contacts connect before signal or power pins, protecting sensitive equipment from voltage spikes.
Mating Cycles
Mating cycles indicate how many times a connector can be connected/disconnected before failure. Cycle ratings vary by connector type (USB, FFC, FPC) based on materials, plating, and mating style. High-cycle connectors suit test equipment and consumer electronics, while low-cycle connectors work for permanent installations.
Mounting
Mounting describes how connectors attach to devices or PCBs. Common methods include PCB (through-hole or surface-mount), edge mount, panel mount, and cable mount. Proper mounting ensures strength, electrical performance, and serviceability in industrial, aerospace, and telecom applications.
Termination
Termination connects wires to a connector's contacts via crimping, soldering, or screw terminals. Each method offers different advantages in mechanical strength and reliability, crucial for signal integrity in cable assemblies and control panels.
Strain Relief
Strain relief mechanisms protect connectors from cable flexing or pulling forces that could damage terminals. These features are vital in industrial automation, robotics, and outdoor installations where cables experience frequent movement.

Performance Parameters
Key performance parameters for evaluating connectors include:
- Current rating – Maximum safe current without overheating
- Voltage rating – Highest voltage without insulation breakdown
- Operating temperature range – Safe temperature limits
- Contact resistance – Electrical resistance at contact interfaces
- Insulation resistance – Prevention of current leakage
- Environmental sealing – Protection against dust/moisture (IP/NEMA ratings)
- Mating force – Insertion/extraction force required
These specifications help select connectors for harsh environments or mission-critical systems.
Features of Electronic Connectors
Specialized connector features include:
- Hermetically Sealed Connectors – Airtight/watertight protection for extreme environments (aerospace, military)
- Water Resistant Connectors – Protection against splashes (consumer electronics, industrial machinery)
- Moisture/Oil Resistant Connectors – Durability in oily/humid conditions (manufacturing, automotive)
- EMI/RFI Filtering Connectors – Signal quality protection (medical, industrial automation)
- ESD Shielded Connectors – Electrostatic discharge protection (microelectronics, test equipment)
Additional features may include polarization, quick-disconnect latches, and overmolded housings for enhanced protection and usability. Choosing connectors with appropriate features ensures reliable performance




