Introduction
This post covers everything you need to know about electric coils available online.
You'll discover:
- The definition of Electric Coils
- Applications of Electric Coils
- Different Types of Electric Coils
- Materials Used in Electric Coils
- Impact of Moisture on Electric Coils
- And much more...
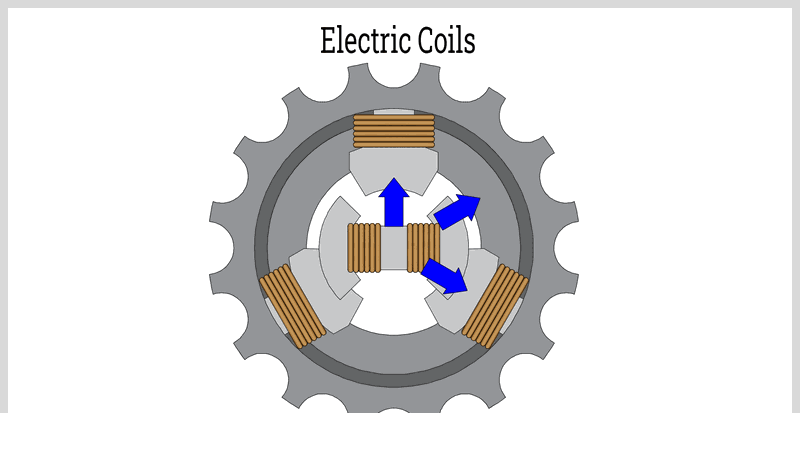
Chapter One – What is an Electric Coil?
An electric coil, or electromagnetic coil, consists of conductive wires wound around a core that may be cylindrical, toroidal, or disk-shaped, typically made of ferromagnetic materials. This essential electronic component provides inductance in circuits, a key property for resisting electrical current.
Available in various types, electric coils differ by wire gauge, diameter, length, loop count, and core material. Core options include air, iron, steel, ceramic, and iron-based amorphous tape, selected based on permeability - the material's ability to enhance magnetic fields effectively.
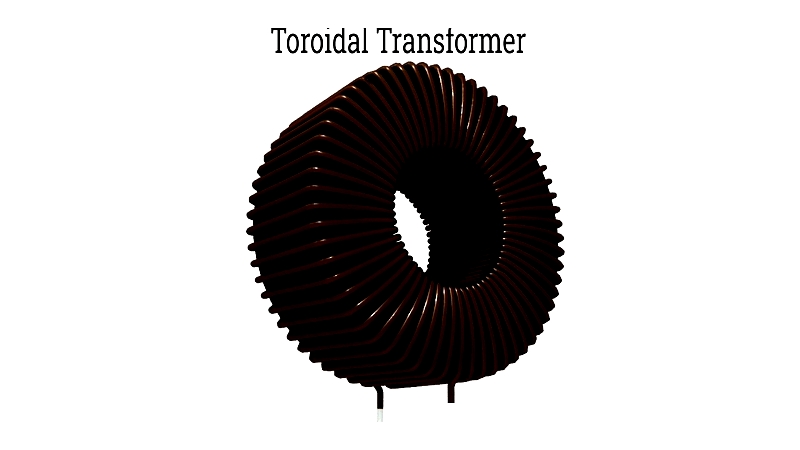
These coils are crucial in devices like motors, generators, inductors, and electromagnets, serving as reliable conductors that deliver steady electrical flow for induction processes.
Chapter Two – How are Electric Coils Used?
Electric coils, also called electromagnetic or induction coils, are fundamental components across numerous industries. They transform, regulate, and control electrical energy in manufacturing, medical equipment, and power distribution systems. Designed for specific applications, coils meet exact electrical, thermal, and mechanical requirements.
Electric Coil Applications:
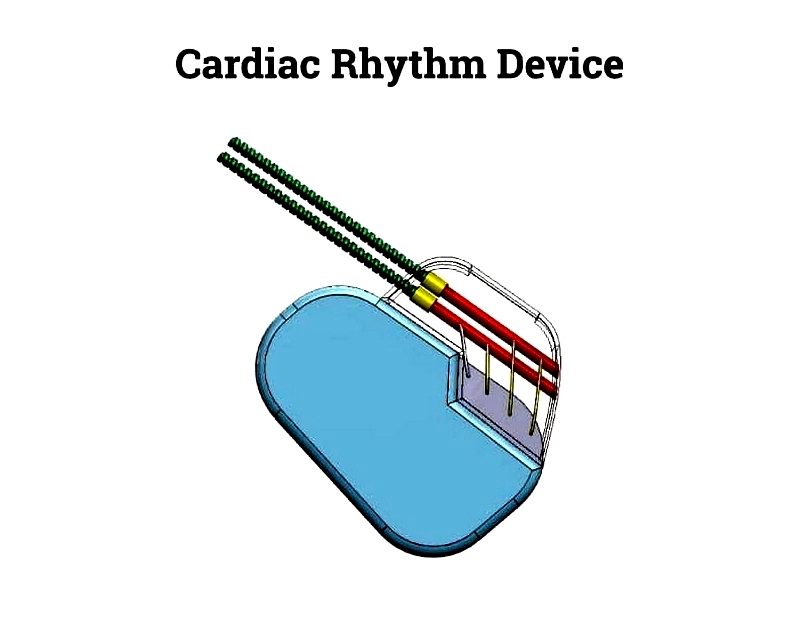
Medical Electric Coils
Medical equipment requires components that meet strict quality and safety standards. MRI coils, solenoid valves, and electromagnetic actuators in medical devices are manufactured in cleanrooms using biocompatible materials with precise tolerances for reliable performance in critical applications.
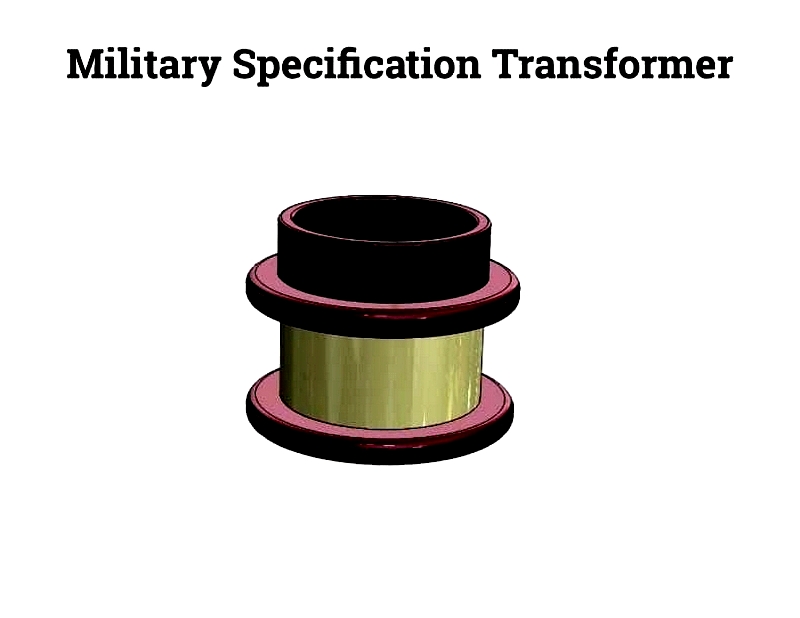
Military Electric Coils
Military-grade coils are built for durability and efficiency in harsh conditions. Used in missile systems, avionics, and radar, they feature low signal loss and EMI resistance to ensure mission readiness.
Transportation Electric Coils
Found in rail systems and vehicles, these coils withstand vibrations and environmental changes. They're used in transformers, braking systems, and signal relays for efficient transit operations.

Energy Electric Coils
Specialized coils in energy sectors endure extreme conditions. Designed for thermal dissipation and insulation, they ensure reliable power conversion in oil platforms, wind farms, and power stations.

Electronic Electric Coils
Miniature coils power consumer electronics, serving as inductors and transformers in devices like smartphones and computers for voltage regulation and signal processing.
Wind Powered Generator Electric Coils
These coils convert wind energy into electricity, built to handle cyclic loads and weather conditions while maximizing output in turbines ranging from 100W to 1MW.
Flowmeter Electric Coils
Used in liquid and gas measurement, these corrosion-resistant coils provide accurate flow readings for industrial automation in harsh environments.
Automotive Electric Coils
Essential for ignition and power systems, automotive coils transform battery voltage for combustion and support electronic features like ABS and diagnostics.
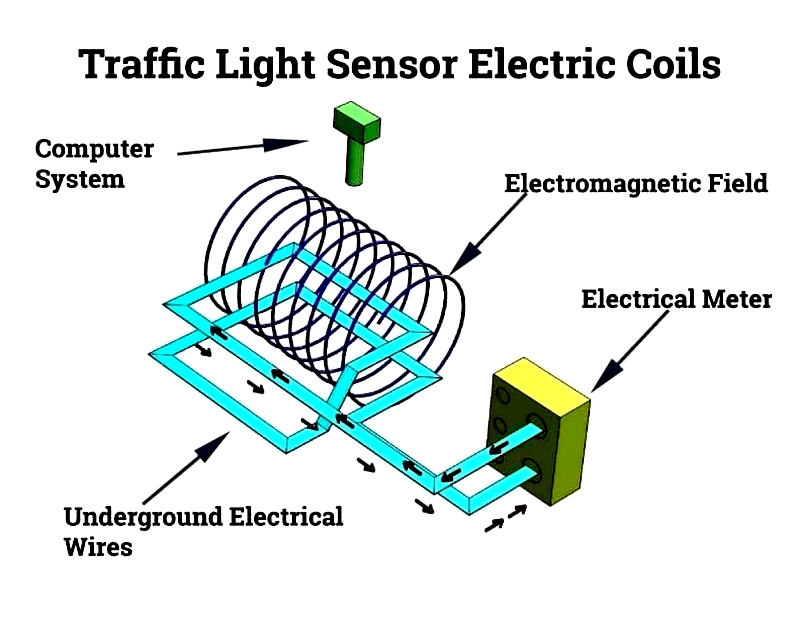
Traffic Light Sensor Electric Coils
Embedded in roads, these coils detect vehicles by measuring inductance changes, optimizing traffic flow through signal adjustments.
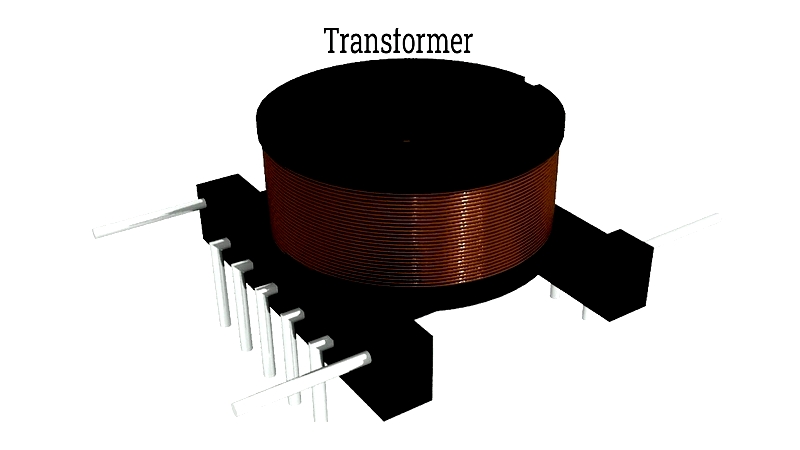
Transformer Electric Coils
Key to power distribution, transformer coils enable efficient AC transmission through electromagnetic induction, reducing energy losses across grids.
Electric Guitar Electric Coils
Pickup coils convert string vibrations into electrical signals, with designs like humbuckers reducing noise while producing distinctive guitar tones.

Choosing Electric Coils: Key Factors
Consider electrical resistance, inductance, thermal rating, winding geometry, insulation, and frequency when selecting coils. Custom solutions may be needed for unique requirements, making manufacturer expertise valuable for quality and compliance.
Understanding coil applications and specifications helps engineers and buyers optimize system performance across industrial, commercial, and consumer electronics.




