Introduction
This article provides comprehensive information about infrared heaters.
Continue reading to learn more about:
- What is Infrared Heating?
- How Infrared Heaters Work
- Different Types of Infrared Heaters
- Benefits of Infrared Heaters
- And More...
Chapter 1: What is Infrared Heating?
Infrared heating uses electromagnetic waves to transfer energy directly to objects without heating the intervening air. The emitted infrared energy typically ranges from 0.7 to 6 microns (µ). By selecting specific wavelengths, energy use is optimized, enhancing heating efficiency.
This method delivers heat directly to materials while maintaining cooler surrounding air temperatures. As a result, infrared heaters are valued for their energy efficiency, convenience, and health benefits. They can operate using electricity, natural gas, or propane, offering an economical and effective heating solution.
Infrared waves span a broad wavelength spectrum, from 780 nm to 10 microns in industrial applications. Shorter wavelengths correspond to higher frequencies and greater energy. Infrared heating can produce temperatures ranging from hundreds of degrees Celsius up to 3,600°C (6,512°F).
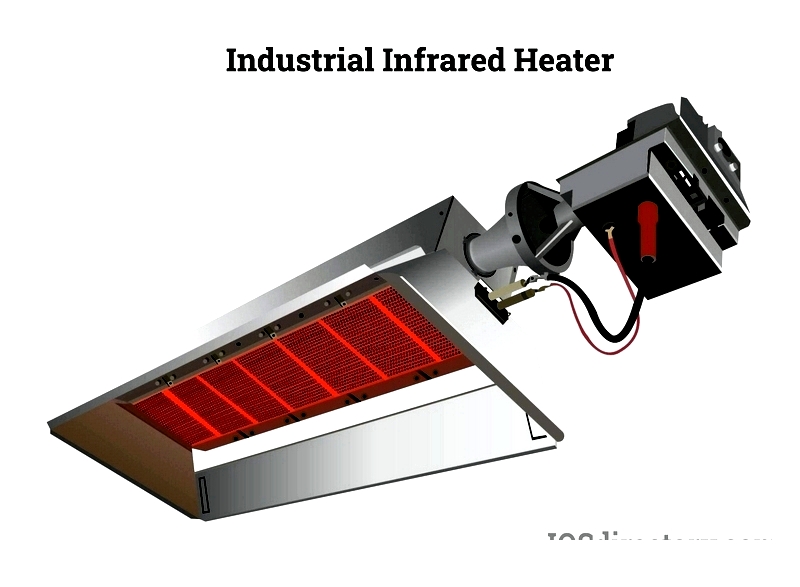
Recent advancements have broadened infrared heating applications. Modern infrared heaters now feature diverse designs and capabilities to meet residential, commercial, and industrial needs. They heat spaces like homes, offices, garages, and warehouses. Industries use them for drying, curing, printing, and thermoforming. In healthcare, infrared heaters aid physiotherapy and rehabilitation.
History of Infrared Heating
British-German astronomer Sir William Herschel first identified the infrared spectrum during the early Industrial Revolution (1760-1840). While the concept was recognized, infrared heating saw limited adoption until World War II, when the military used it for drying paints and lacquers on equipment. This proved more efficient than fuel-intensive convection ovens.
Infrared heaters became common in wartime factories but declined post-war as central heating systems gained popularity.
With growing environmental awareness, infrared heater development accelerated from the late 20th to early 21st century. Applications expanded significantly during this period. Design improvements enabled infrared heater installation in diverse settings, from homes to industrial plants. Technological advances and better controls continue to drive infrared heating's evolution and adoption.
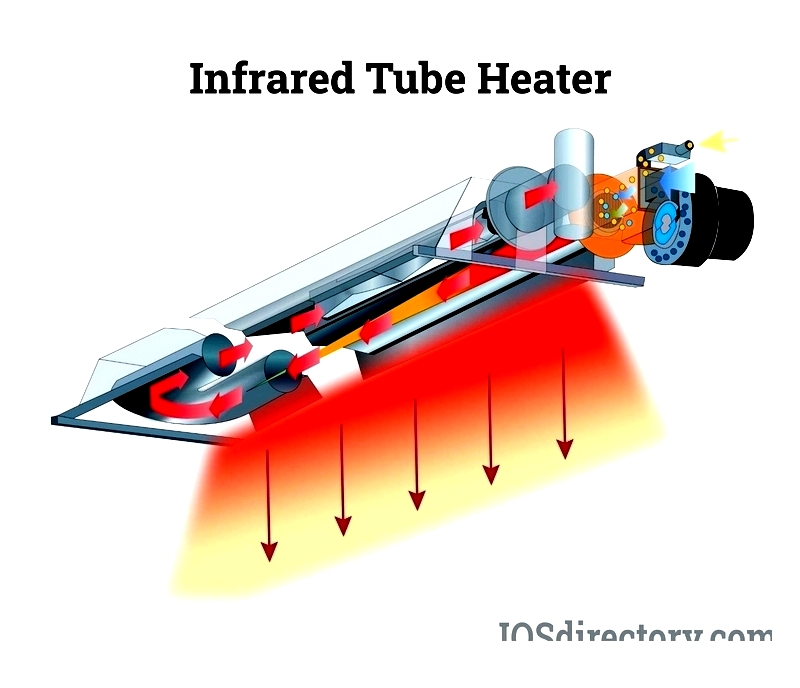
Chapter 2: How Do Infrared Heaters Work?
Infrared heat represents a form of radiant heating that transfers thermal energy directly from heater to object, bypassing air heating. This mimics how sunlight warms Earth, delivering natural, efficient warmth to people, surfaces, and workspaces. Consequently, infrared heating has grown popular for residential and industrial uses like space heaters, patio heaters, and industrial process heating.
Infrared heaters contain specialized heating elements that emit infrared radiation when heated. This radiation travels through air until striking objects like furniture or people, transferring heat directly. Similar to radiant heat transfer between metals via electromagnetic waves, this ensures rapid, targeted heating with minimal energy loss compared to convection systems.
Unlike conventional heaters that warm air first, infrared heaters project radiant heat directly to surfaces. This enables infrared systems to quickly raise surface temperatures efficiently. Users benefit from energy savings, lower costs, better air quality, and consistent warmth, making infrared heaters cost-effective solutions for various heating needs.
Electromagnetic Waves
Electromagnetic waves consist of perpendicular oscillating electric and magnetic fields. These waves facilitate infrared energy transfer and are fundamental to all infrared heating devices.
Characterized by wavelength and frequency, these waves are measured in nanometers or angstroms. Frequency, measured in Hertz, categorizes electromagnetic waves including ultraviolet, visible light, and infrared.
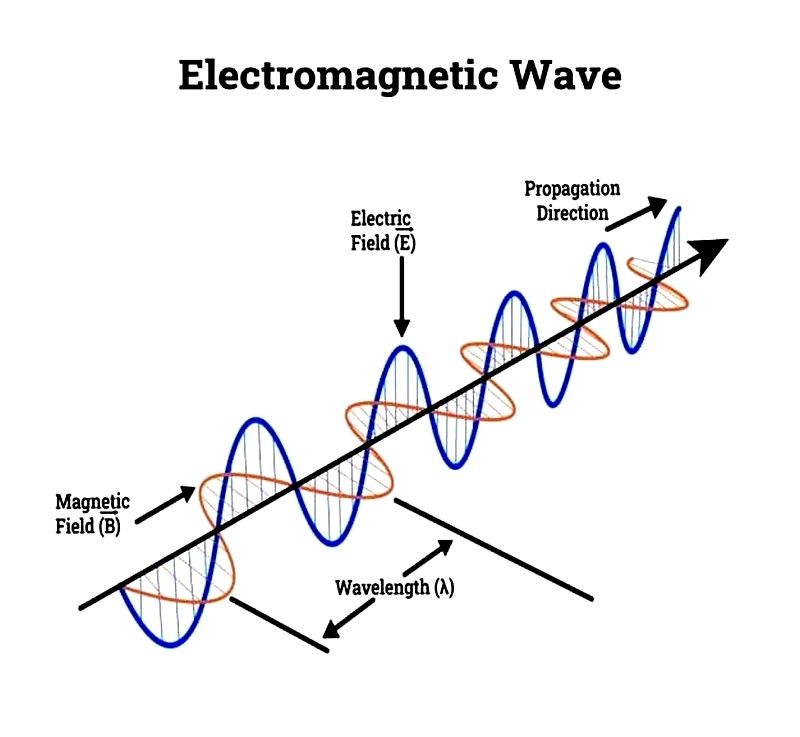
Wavelength and frequency share an inverse relationship: higher frequency means shorter wavelength. Higher-frequency waves carry more energy, making them more effective for heat transfer. Unlike mechanical waves requiring physical media, electromagnetic waves like infrared can travel through vacuum, explaining how sunlight's heat reaches Earth. Infrared heaters apply these principles for efficient indoor/outdoor heating.
Infrared Waves
Infrared radiation occupies the electromagnetic spectrum between visible light and microwaves, spanning 700 nm to 1 mm wavelengths. This range enables diverse heat transfer applications.
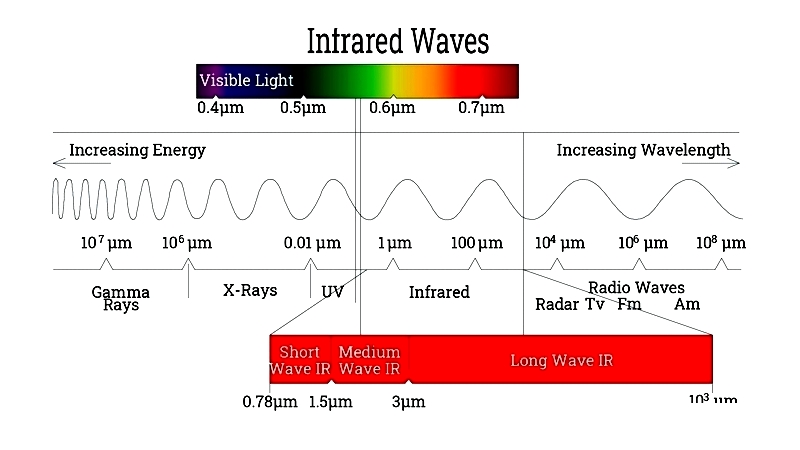
The infrared spectrum includes near-infrared (NIR) for fast industrial processes, mid-wave (MWIR) for drying, and far-infrared (FIR) for comfort heating. Below is a classification table:
(3591-1797°C)
(1797-693°C)
(693-89°C)
(89 - −80°C)




