Introduction
This article explores abrasive blasting techniques and applications.
It covers the following key topics:
- Abrasive Blast Equipment
- Types of Abrasive Blast Systems
- Components and Performance Specs for Abrasive Blast Machines and Sandblasters
- Tumble Blasting
- Sandblasting
- Abrasive Blasting
- Abrasive Blast Rooms
- Benefits of Implementing Blast Systems

Chapter 1: Applications of Abrasive Blast Equipment
Abrasive blasting serves multiple industries with applications including rust and oil removal, paint stripping, surface preparation for coatings, metal surface enhancement, and stone carving.
This technique propels abrasive materials at high velocity to clean, smooth, or shape surfaces. Developed over 150 years ago, it remains a preferred method due to its efficiency and versatility.

Commonly called sandblasting, the process also goes by media blasting or grit blasting. Compared to sanding or chemical stripping, it offers superior cost and time efficiency.
Selecting appropriate abrasive materials is crucial for achieving optimal surface finishes. The anchor pattern created directly affects coating adhesion, making media selection vital for surface preparation.
Abrasive Blasting Media Characteristics
Media size, shape, hardness, and density significantly influence surface outcomes.

Size: Larger particles create deeper impressions than smaller ones. Abrasives are classified by mesh size, grit size, or microns.
Mesh size indicates particles per square inch on a sieve. Grit size similarly measures particle retention. Microns provide metric measurements for fine abrasives.
Shape: Media shape affects cutting penetration. Angular media with sharp edges create deeper profiles, while rounded shapes produce smoother surfaces.
Hardness: Measured on the Mohs scale, harder abrasives like aluminum oxide (9) create deeper profiles than softer materials like plastic abrasives (3-4).
Density: Denser particles create deeper profiles with less deformation. Specific gravity measurements indicate density differences between media types.
Abrasive blast systems effectively clean machine parts, remove deposits, and prepare surfaces for treatments. They handle components like dies, molds, and castings while removing scale, rust, and coatings.
The process enhances surfaces for plating, painting, and coating adhesion. Peening improves fatigue resistance and stress relief at welds. Industries from automotive to jewelry benefit from the superior finishes.
Various media including aluminum oxide, glass beads, and steel grit are selected based on desired outcomes and material requirements.
Chapter 2: Types of Abrasive Blast Systems
Selecting the appropriate abrasive blast system is essential for industrial surface preparation. Different industries require specialized equipment to handle various materials and project scales.
Abrasive Blast Cabinets: These enclosed systems clean precision parts using controlled abrasive flow. Ideal for workshops and labs, they minimize media loss and dust exposure.

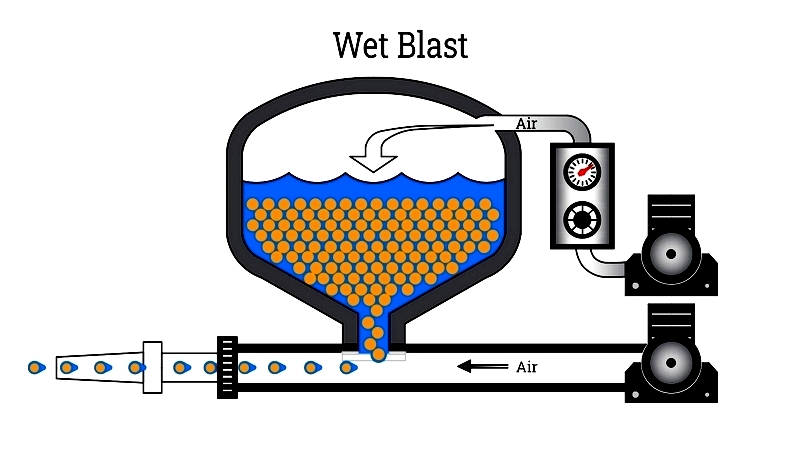
Wet Blast: Combining water with abrasives, this method reduces dust while cleaning and preparing surfaces. It's particularly effective for rust removal and delicate components.
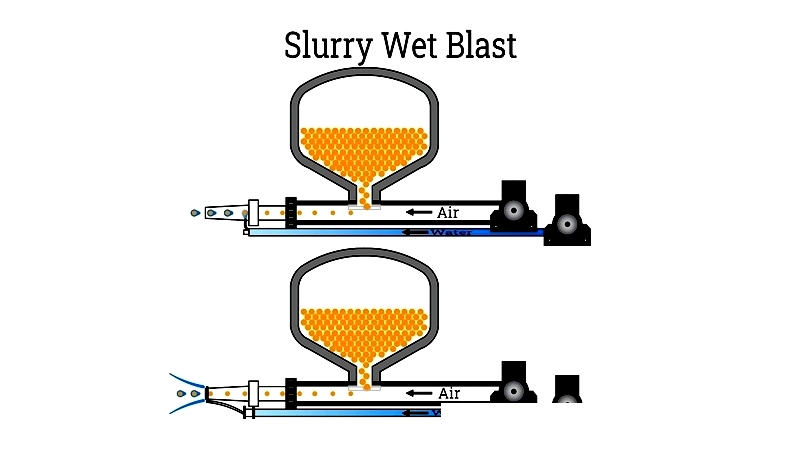
Slurry Blast: Using water-abrasive mixtures, this system quickly removes contaminants while producing uniform finishes. It's valuable for heavy machinery and marine applications.
Vacuum Blast: This closed-loop system collects abrasives during blasting, reducing environmental contamination. Ideal for hazardous material removal and confined spaces.

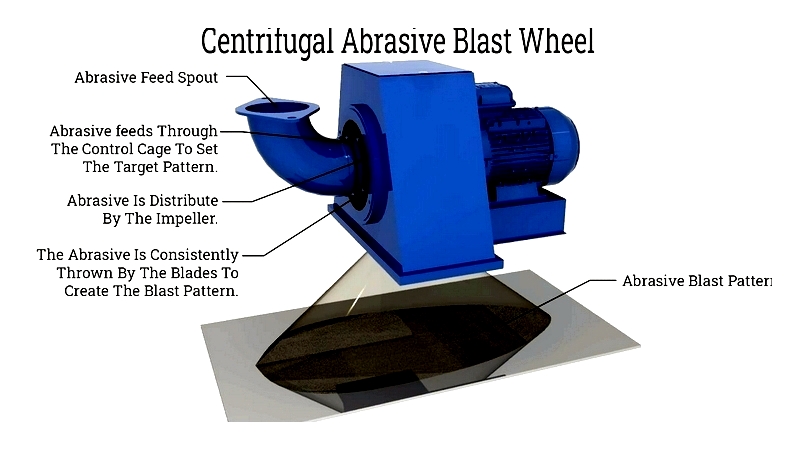
Centrifugal Blast: Using high-speed wheels, this method provides uniform cleaning for large-scale operations like structural steel preparation.
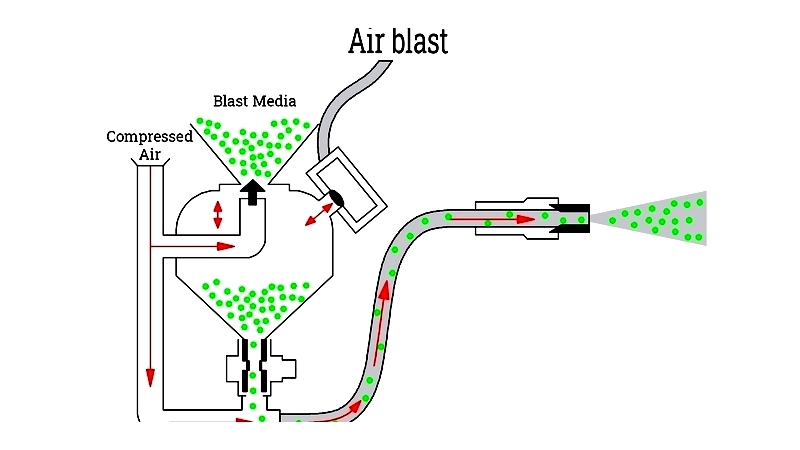
Air Blast: The most versatile method, using compressed air to propel dry abrasives. Suitable for diverse materials from steel to glass.
Bristle Blast: This mechanical method uses rotating wire brushes to clean surfaces without significant material loss, ideal for weld cleaning and corrosion removal.
Pencil Blast: A precision technique for delicate components, using fine abrasives to remove micro-contaminants from intricate parts.
Choosing the right system requires considering substrate type, desired finish, environmental factors, and safety requirements. Modern systems incorporate dust control and media recycling for improved efficiency and compliance.




