Introduction
This article provides an in-depth examination of Friction Materials.
You will explore topics including:
- What are Friction Materials?
- Applications of Friction Materials
- Manufacturing Process of Friction Materials
- Varieties of Friction Materials
- And more…
Chapter One – What are Friction Materials?
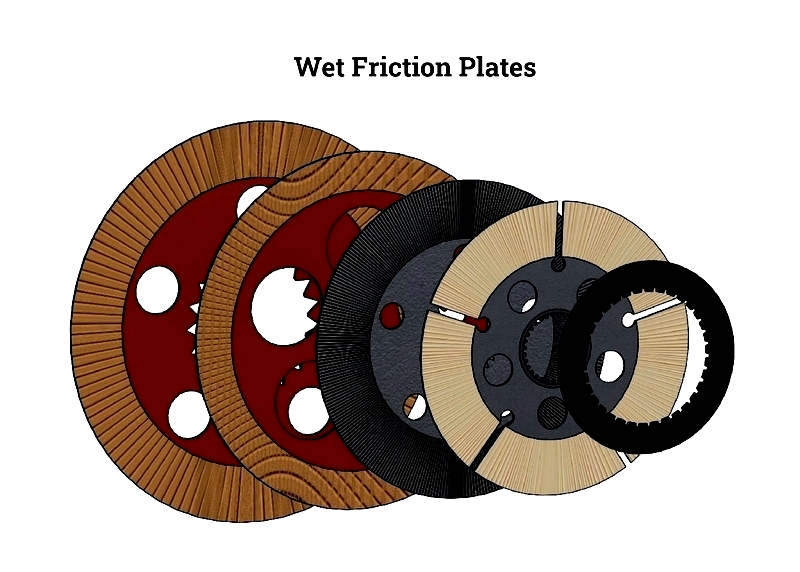
Friction materials are specially engineered substances designed to create friction between surfaces, allowing for motion control or stoppage. Composed of both organic and inorganic elements like resins, ceramics, fibers, and metals, these materials are crucial in numerous applications. Given their functional nature, friction materials have a limited lifespan and require regular inspection and timely replacement.
Primarily used for effective braking and power transmission while minimizing wear, friction materials appear in common products such as brake pads, brake shoes, clutch plates, bonded assemblies, friction bands, liners, and rolls. They come in various forms including bands, blocks, pads, discs, rolls, sheets, and linings.
Among the wide range of friction materials, ceramics stand out for their durability and ability to withstand heavy loads. Other notable friction materials include metals, rubber, resins, aramid fibers, and graphite, each offering unique properties for specialized applications.
The transportation industry is the primary user of friction materials, particularly in braking systems, clutch mechanisms, and vehicle transmissions. Since their introduction, these materials have been fundamental to numerous product innovations.
Chapter Two – What are the uses for friction materials?
Friction, the resistance to motion when two surfaces interact, is essential across industrial, automotive, and mechanical systems. Friction materials leverage this property, making them indispensable for applications requiring precise braking, reliable stopping, smooth acceleration, and controlled directional changes. These advanced materials not only stop, slow, or regulate movement but also establish secure mechanical connections between components. Consequently, friction materials are critical for the performance, reliability, and safety of brake and clutch systems, industrial machinery, and transportation equipment.
Available in various types, grades, and configurations—including organic, semi-metallic, sintered, and ceramic formulations—friction materials offer versatile solutions for diverse applications. Each type's function, durability, and wear resistance depend on its unique composition and design, tailored for optimal performance in specific environments.
Friction Disc
Friction discs, also called clutch discs or brake discs, are key components in disc brake systems for vehicles, trucks, industrial presses, and heavy equipment. They provide consistent stopping power by transmitting torque and converting kinetic energy into heat through friction. Made by attaching specialized friction compounds like ceramic, carbon, or semi-metallic materials to metal backing plates with adhesives or rivets, friction discs offer high heat resistance, stable wear, and superior performance under repeated braking. This makes them ideal for demanding automotive and industrial applications with frequent starts, stops, and heavy loads.

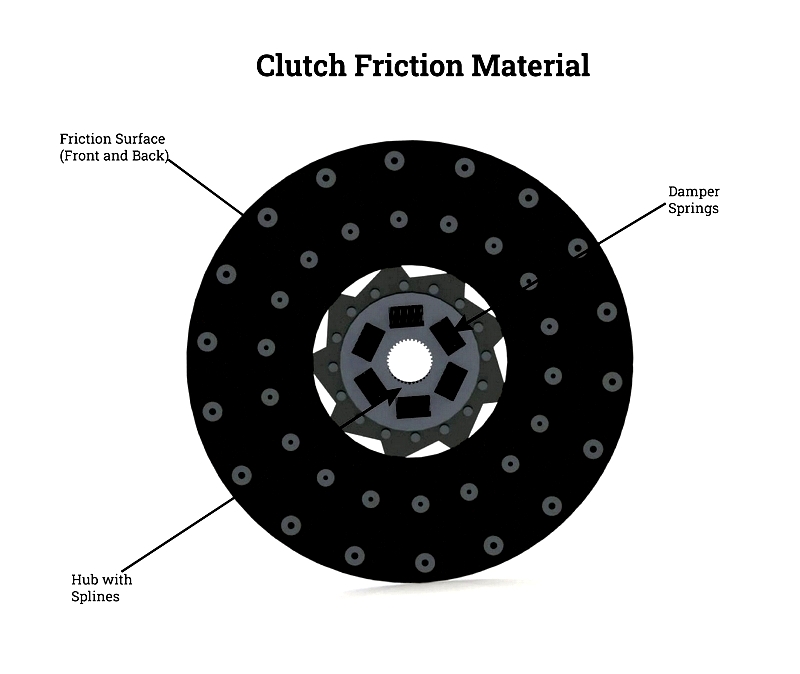
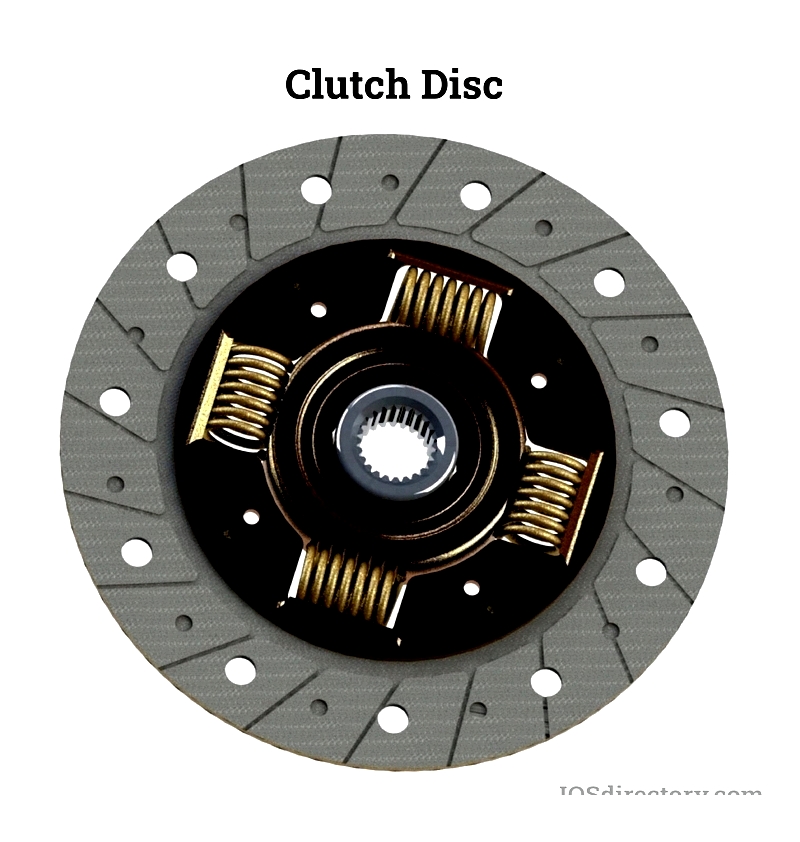
Clutch Disc
The clutch disc, or clutch lining, is a vital part of automotive and industrial clutch assemblies. Attached to the transmission shaft, it connects with the engine flywheel when the clutch pedal is engaged, enabling smooth gear shifts and controlled acceleration or deceleration. Found in trucks, buses, manufacturing presses, and CNC machinery, clutch discs are made from asbestos-free materials like organic resins, aramid fiber, or copper compounds, balancing noise reduction, durability, heat dissipation, and torque transfer.
Slip Clutch
A slip clutch, or back torque limiter, protects PTO shafts, gearboxes, and drivelines by allowing controlled slippage during torque overloads. This prevents mechanical damage and downtime. Compact in design, slip clutches feature pressure plates, friction plates, torsion springs, and adjustment mechanisms to calibrate slip torque. Used in shaft-to-shaft, shaft-to-pulley, and gear couplings, they disengage the drive when torque exceeds preset values, protecting systems during jams or emergency stops. Common in agricultural machinery, construction equipment, and motorcycles, slip clutches enhance safety and equipment longevity.
In agriculture and industry, slip clutches manage shock loads, synchronize speeds, and ensure smooth transitions, protecting PTO shafts and extending the life of tractors, balers, and conveyors.
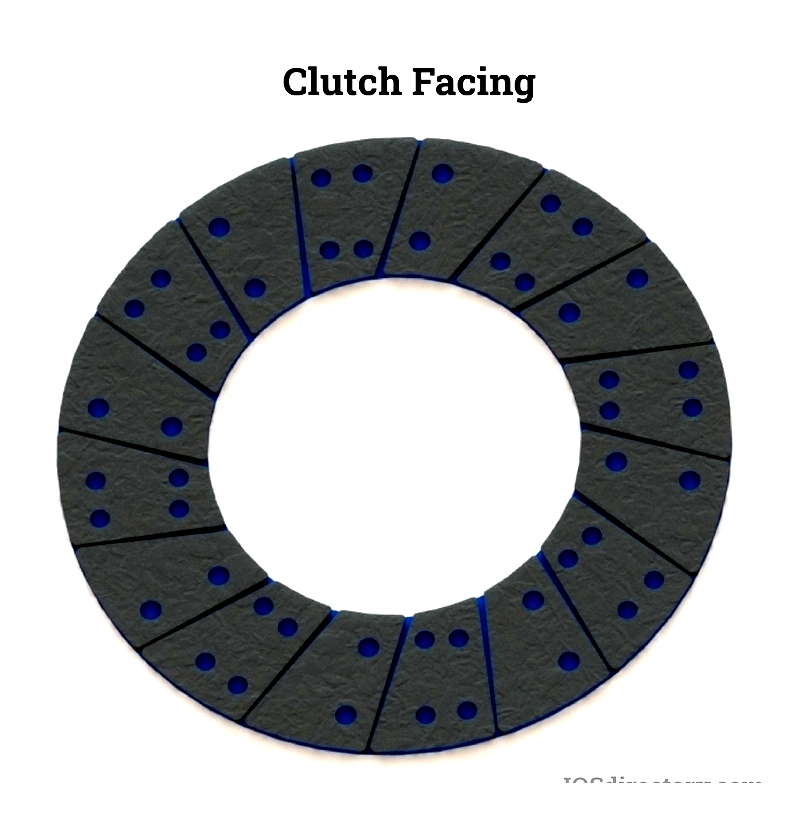
Clutch Facing
Clutch facings are high-friction linings on clutch discs that reduce noise and vibration during engagement. Engineered for smooth operation under high speeds and temperatures, modern facings use composite materials like non-asbestos organics, metallic blends, and aramid fibers for heat resistance and durability. These facings improve performance in automotive, forklift, marine, and industrial systems by resisting thermal degradation and mechanical stress.
Brake Pads
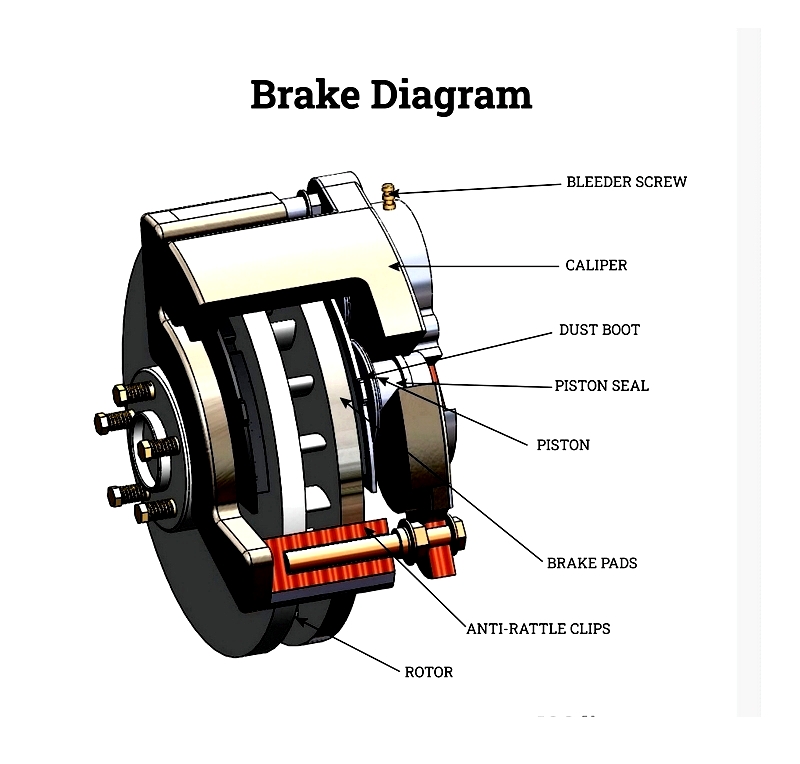
Brake pads are essential in disc brake systems for cars, motorcycles, trucks, and industrial equipment. Made from semi-metallic, non-asbestos organic (NAO), or ceramic materials, they offer distinct advantages. Semi-metallic pads excel in heat dissipation and heavy-load performance, NAO pads provide quiet operation with low dust, and ceramic pads offer minimal noise, rotor wear, and fade resistance. Historically, drum brake shoes were common, but disc brakes and pads now dominate due to better heat management. Selecting the right pad material is crucial for specific needs like towing, racing, or heavy-duty hauling.
Brake Lining
Brake linings enhance braking efficiency by optimizing friction and reducing metal-to-metal wear. Made from ceramics, aramid, metallic fibers, and resins, they minimize noise, prevent fade, and withstand thermal cycling. Used in brake pads and drum brake shoes, linings are versatile across automotive, industrial, and railway applications.
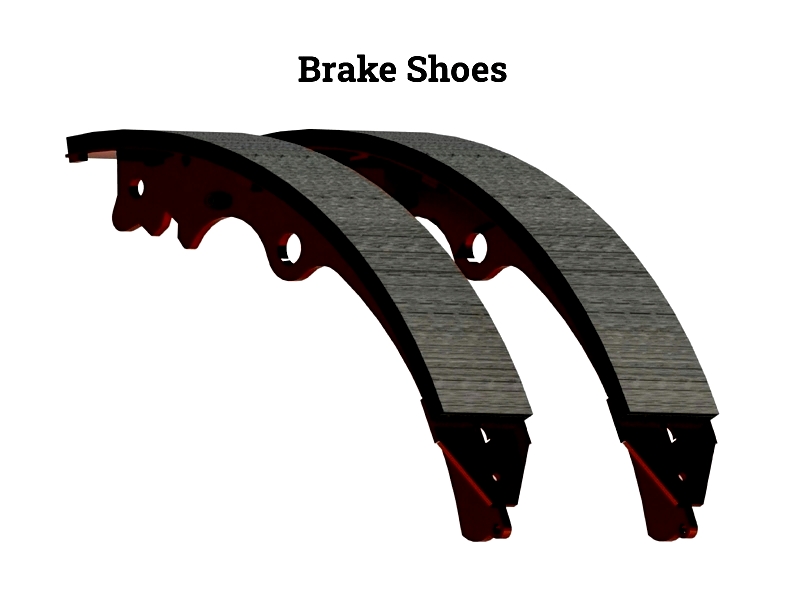
Brake Shoes
Brake shoes are key in drum brake systems, using friction linings to press against the drum's inner wall. Unlike disc brake pads, shoes expand outward to create braking force. Essential for trucks, trailers, and industrial machinery, modern brake shoes balance friction, wear, and noise reduction for durability in harsh conditions.
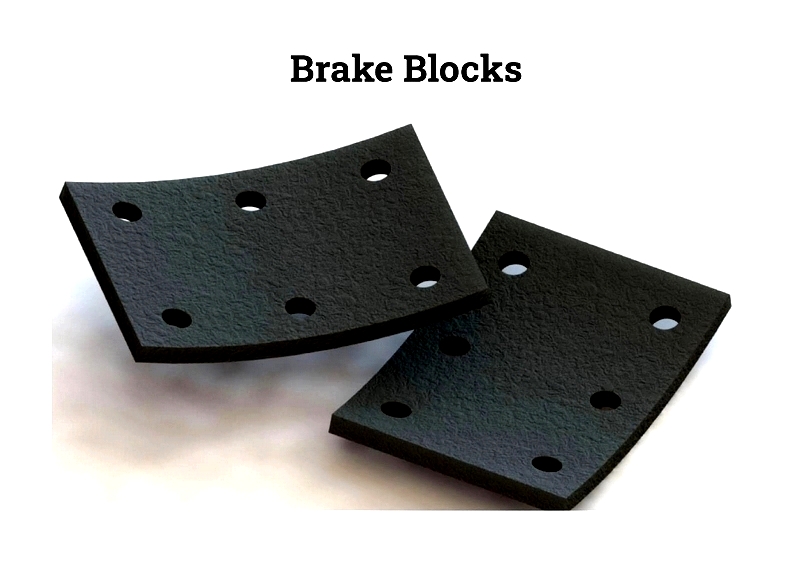
Brake Block
Brake blocks, made from rubber, composites, or resins, directly apply friction to wheel treads in bicycles, cranes, and industrial machines. They provide reliable stopping power and are secured with rivets, screws, or adhesives. Advanced compounds improve heat dissipation, noise reduction, and durability in railroads, elevators, and heavy machinery.