Introduction
This article provides a comprehensive guide to pneumatic solenoid valves. Continue reading to learn about:
- What gear drives are
- The functions of gear drives
- Different types of gears
- Common gear drive applications
- And more...
Chapter 1: What are Gear Drives?
Gear drives, also known as gear trains or gearboxes, consist of gears, shafts, and other components designed to securely connect rotating parts. Their main purpose is to transfer power from a driving source (such as an engine, turbine, or motor) to a driven machine. Different gear arrangements allow gear drives to adjust the transmitted power as needed.

Gear drives can modify the rotational speed of the output shaft, either increasing or decreasing it. A common application is reducing motor and engine speeds, which typically operate at high revolutions per minute (rpm). These systems are called speed reducers. Lowering the speed increases torque, which is a key feature of speed reducers.
Gears are the core components of gear drives. These toothed elements interlock by meshing with each other. Due to the significant forces they endure, gears are usually made from alloyed steel. Heat treatment enhances these metals to provide the necessary strength and rigidity for specific applications.

Gear drives also include additional components like shafts, keys, couplings, bearings, housing, and flanges. Shafts connect the gear drive to input and output devices. Keys and couplings secure the driving and driven shafts to the gear drive. Bearings support the shafts to minimize friction. The housing and flanges are typically constructed as a single unit, with the housing enclosing and stabilizing the assembly while flanges assist with mounting.
Chapter 2: Functions of Gear Drives
Gear drives are essential mechanical power transmission systems used in various industrial and commercial applications. They are fundamental for controlled motion, efficiency, and reliable torque transfer. Common applications include automotive transmissions, wheel differentials, marine propulsion systems, industrial machinery, wind turbines, and electric gear motors. Compared to other power transmission methods like belt or chain drives, gear drives offer superior efficiency, precise speed control, heavy load capacity, compact design, and durability. In industrial automation, robotics, material handling, and renewable energy sectors, gear drives enable high-performance operations and energy efficiency. The following sections describe their primary functions.
Changing the Speed of Rotation
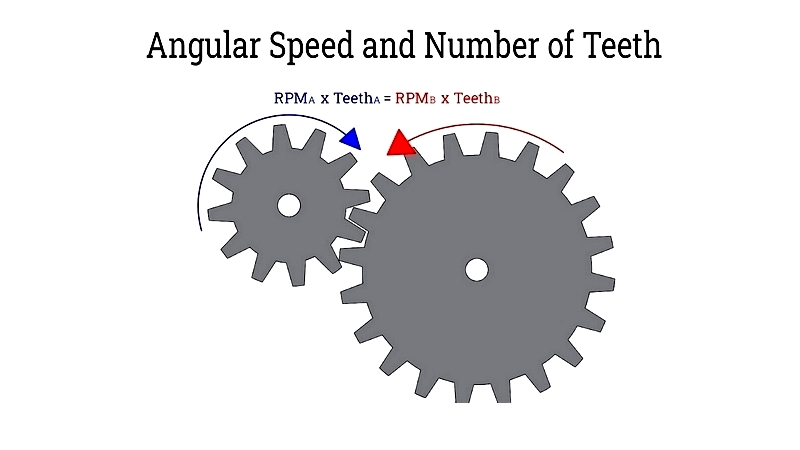
A core function of gear drives is modifying the rotational speed of the driven shaft relative to the driver. By using gears of different sizes or tooth counts, gear drives achieve the desired output speeds. For example, a larger driver gear meshing with a smaller driven gear increases speed, while the opposite configuration reduces speed and increases torque.
This principle is based on the constant linear velocity at the pitch circles of meshing gears. The relationship is expressed by the formula where v is linear speed, ra and rb are gear radii, and ωa and ωb are angular velocities:
The gear ratio, a key concept in power transmission, represents the proportion between the teeth or pitch diameters of the driver and driven gears. This ratio determines how speed and torque are adjusted in transmissions and gearboxes. The relationship is defined below, where da and db are pitch diameters, and Na and Nb are tooth counts:
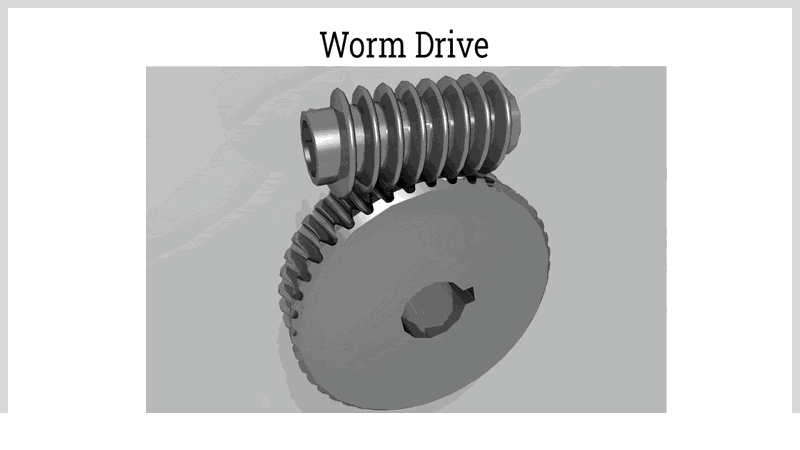
Different gear types, such as spur, helical, bevel, and specialty configurations, allow for tailored speed control. Worm drives, consisting of a worm and worm wheel, offer high reduction ratios and smooth operation. They are commonly used in conveyor systems and lifts, where significant speed reduction is needed. Worm drives also prevent back-driving, adding a safety feature.
Planetary gear drives use a central sun gear, orbiting planet gears, and a surrounding ring gear. This compact arrangement provides multiple speed and torque combinations, making it ideal for robotics, vehicle transmissions, and industrial machinery.
When selecting a gear drive, consider factors like gear ratio, speed requirements, torque capacity, efficiency, and maintenance needs to ensure optimal performance.
Increasing or Decreasing the Output Torque
Gear drives can adjust output torque as needed. Increasing speed reduces torque, while decreasing speed increases torque. This principle is valuable in heavy machinery, cranes, and industrial equipment where precise force delivery is critical.
based on energy conservation, the power (P) transmitted through a gear drive remains constant, with τa and τb representing torques:
The mechanical advantage (MA) quantifies how input force is converted to output torque. Engineers select gear trains to optimize torque for demanding applications like mining equipment or wind turbines.
To adjust torque, designers may modify tooth counts, use multiple gear stages, or select durable materials and lubrication systems for long-term reliability.
Modifying the Axis of Rotation
Gear drives can change the axis of rotation between components, which is essential in manufacturing, automotive, and aerospace systems.
- Offsetting the output shaft while keeping it parallel to the input shaft
- Changing the axis angle using bevel gears, as in differential systems
- Creating non-parallel, non-intersecting shafts with hypoid or worm gears
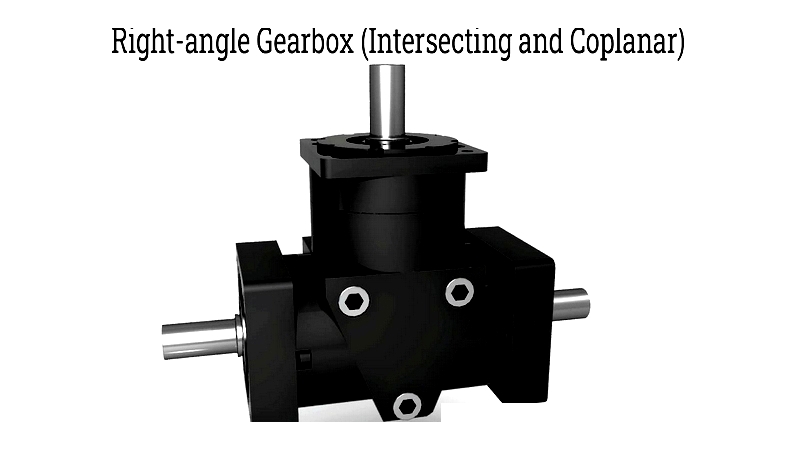
While spur and helical gears work with parallel shafts, specialized gears like worm and bevel gears enable versatile shaft configurations. Hypoid gears offer quiet operation for high-load applications like automotive axles.
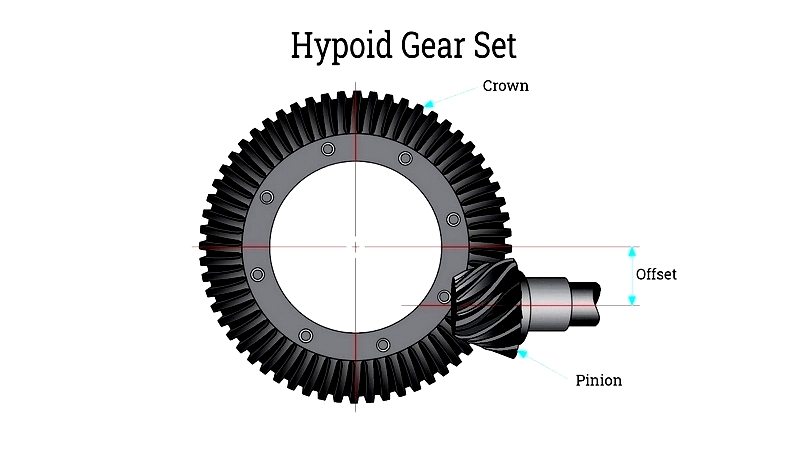
Gear selection and design are crucial for meeting spatial and mechanical requirements in advanced machinery.
Reversing the Direction of Rotation
Gear drives can reverse rotational direction, which is useful in automotive transmissions and industrial equipment. In a two-gear system, the gears rotate in opposite directions. More complex arrangements can maintain or reverse output rotation.
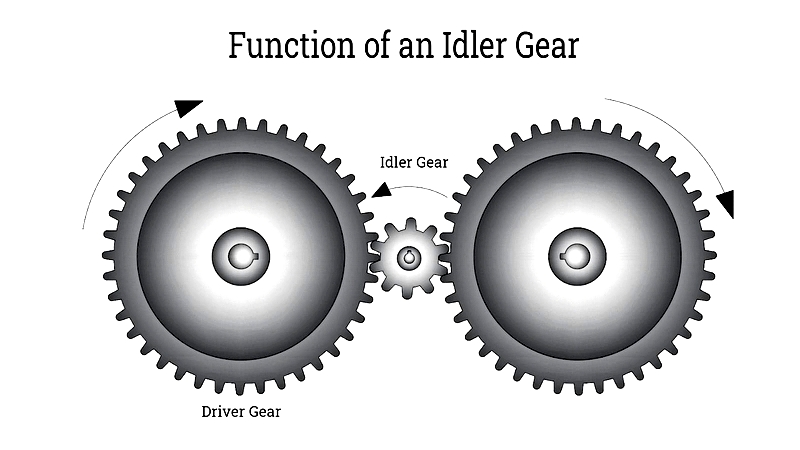
Idler gears allow direction reversal without affecting gear ratio or torque. This is valuable in manual transmissions and manufacturing systems requiring bidirectional motion.
When selecting




