Introduction
This article provides detailed information about welded wire mesh.
It covers the following topics:
- What is welded wire mesh?
- Types of welded wire mesh
- Benefits and applications of welded wire mesh
- Galvanized wire mesh manufacturing methods
- Galvanized steel mesh panels
- Types of galvanized steel mesh panels
- Applications and benefits of galvanized steel mesh panels
- And more...
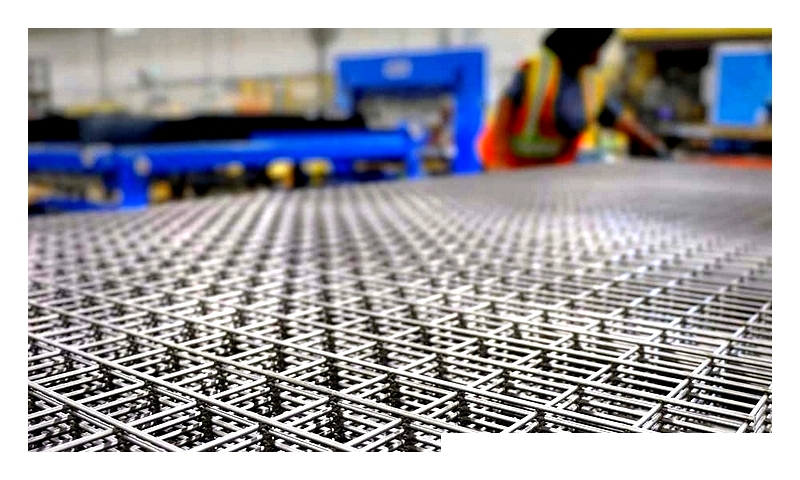 Welded Wire Mesh by Banker Wire
Welded Wire Mesh by Banker Wire
Chapter 1: What is Welded Wire Mesh?
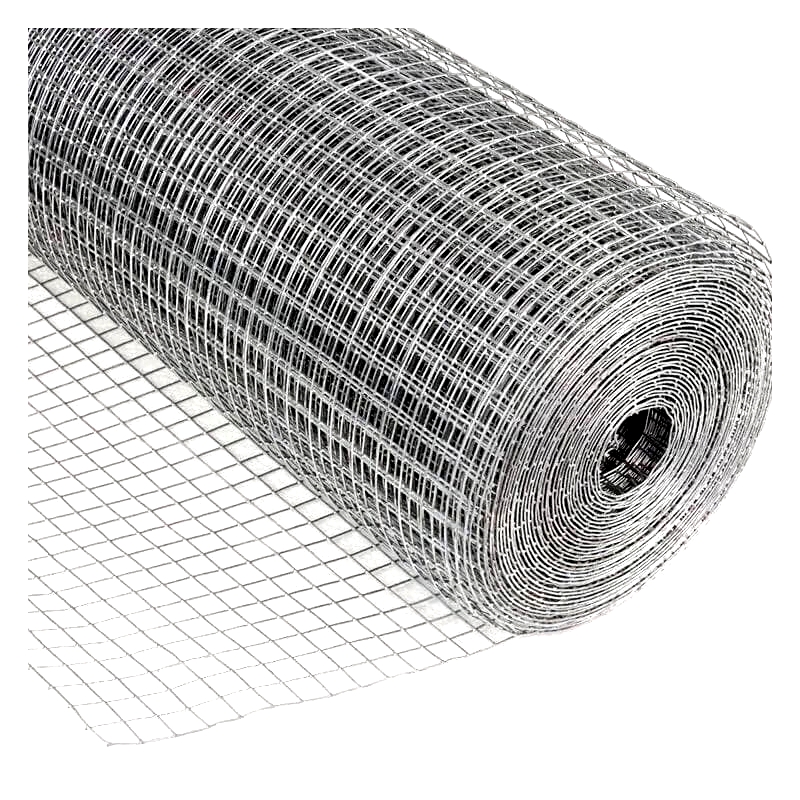
Welded wire mesh consists of interconnected wires fused at their intersections. Mesh openings vary depending on the wire type and intended use. Regardless of wire size or type, this mesh is exceptionally strong and requires significant force to dismantle.
The production process involves feeding wire spools into a welding machine. This machine uniformly welds wire intersections at multiple points simultaneously, ensuring efficiency and reliability.
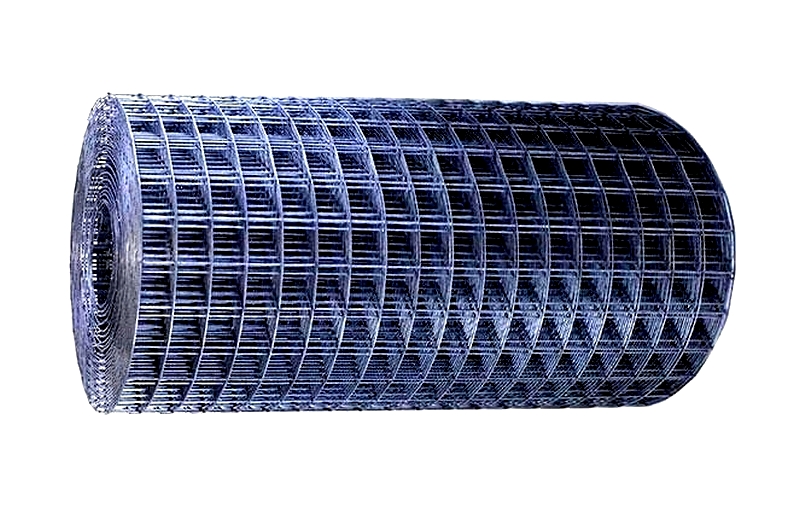
Commonly known as "weldmesh," it's available in sheets or rolls. Thinner wires allow for larger openings while maintaining structural integrity. It's typically made from mild steel, galvanized steel, or stainless steel.
In construction, mild steel is commonly used for retaining walls and structural reinforcement. Galvanized mild steel is popular for fences, security screens, partitions, storage solutions, machine guards, cages, and aviaries. It's made from pre-galvanized or hot-dipped wire, with hot-dipping preferred for its cleaner finish and ability to conceal welds.

Stainless steel welded mesh is ideal for food and pharmaceutical industries due to its hygiene standards and resistance to harsh conditions and rust.
Unlike woven mesh where openings refer to space between wires, welded mesh openings are measured center-to-center. When purchasing, specify material, center-to-center spacing (or clear opening), wire thickness, dimensions, and quantity.
Chapter 2: Types of Welded Wire Mesh
Welded wire mesh, also called welded wire fabric, is fundamental across industries due to its durability, high tensile strength, and versatility. Known for maintaining shape and structural integrity, it's used in perimeter fencing, animal cages, industrial shelving, machine guards, grates, and partitions. Selecting the right type is crucial as each application requires specific mesh configurations to meet performance, safety, and environmental needs.
Technically, it's a prefabricated grid of longitudinal and transverse wires—typically made from carbon steel, galvanized steel, or stainless steel. These wires are welded at intersections via electric resistance welding, ensuring stability, uniform openings, and minimal wire movement during use.
Square Welded Wire Mesh
Square welded wire mesh features wires intersecting at right angles, creating uniform openings. This pattern provides even load distribution and increased strength, making it ideal for machine guards, fencing, decorative panels, and protective enclosures. Available in various sizes, thicknesses, and finishes like hot-dip galvanized and 304 or 316-grade stainless steel, it meets both aesthetic and performance requirements.
Rectangular Welded Wire Mesh
Similar to square mesh but with elongated rectangular openings, this type offers strength along the wider axis and better control of ventilation or visibility. Rectangular welded wire mesh is used in poultry cages, security partitions, construction reinforcements, and conveyor safety screens where strength, airflow, and visibility are balanced.
PVC Welded Wire Mesh
PVC welded wire mesh features steel or galvanized wire coated with polyvinyl chloride (PVC) for advanced corrosion resistance. The coating adds color and protects against rust, weathering, chemicals, and UV rays. PVC-coated welded wire mesh is widely used in agricultural fencing, animal enclosures, property boundaries, landscaping, and temporary barriers. Its flexibility absorbs shocks, reduces maintenance, and increases longevity for indoor and outdoor use.
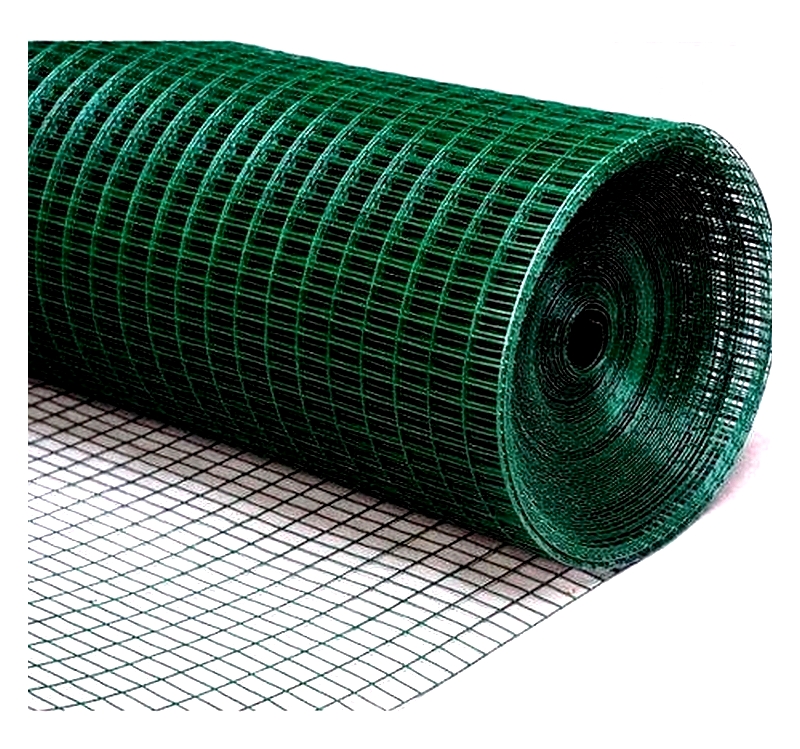
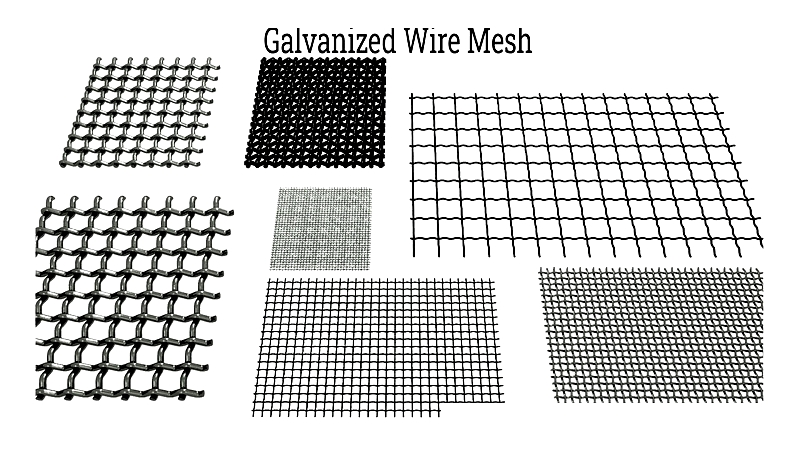
Galvanized Welded Wire Mesh
Galvanized welded wire mesh features a protective zinc coating for superior rust and corrosion resistance in harsh environments. Available through electro-galvanizing or hot-dip galvanizing (the latter offering better durability), it's used for security fencing, animal pens, equipment guarding, and infrastructure reinforcement. Its longevity makes it ideal for agricultural, industrial, and marine applications.
Welded Stainless Steel Wire Mesh
Made from corrosion-resistant grades like 304 or 316 stainless steel, this mesh offers durability, hygiene, and chemical resistance. It's used in filtration screens, food processing, chemical processing, architectural facades, and pharmaceutical production. Its non-reactive nature makes it ideal for contaminant-free applications, available in panels, rolls, or custom shapes.
Welded Wire Fencing
Known for strength and security, welded wire fencing comes in rolls or panels, available in galvanized or untreated steel. It's ideal for residential fencing, commercial barriers, pet enclosures, and sports field perimeters. Easy to install and adaptable, it's a cost-effective alternative to chain link fencing.

Installation requires posts, rings, and pliers. Pre-fabricated panels allow quick setup and disassembly for temporary barriers.
Constructed from thick gauge steel, modifications may need power tools. Combined with concrete footings and reinforced posts, these fences provide stability for high-security areas. Accessories like barbed wire can be added for extra protection.





